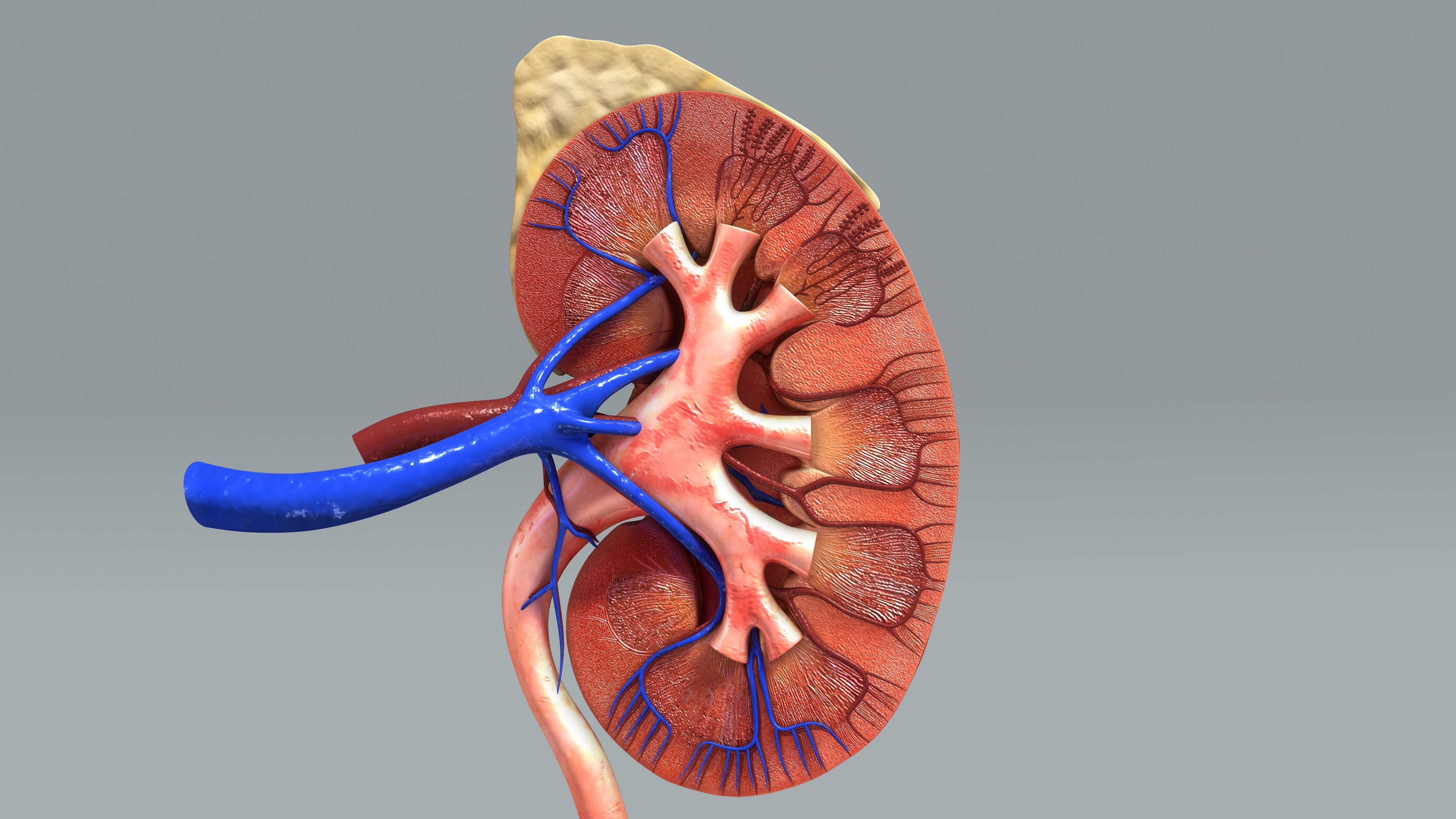
Verici Dx (VRCI ) said its two leading products, Clarava™ and Tuteva™, have now completed the testing requirements of a multicentre validation study ‘in line’ with previous expectations.
The Company, which develops advanced clinical diagnostics for organ transplants, previously told investors that it expected the validation study to be completed prior to the end of 2021.
Verici said that both assets had not only exceeded its objectives for the study in terms of numbers of sites and participants, but also completed testing in line with its expectations.
Reaching the clinical endpoints of the study means that, for both products, ‘sufficient numbers of participants have completed the required follow-up to outcomes assessment,’ it explained.
In practice, this includes biopsy findings, which are required for the full data analysis and clinical study report to be conducted in Q1 2022, as per the Company’s targets, it noted.
Verici’s Clarava™ assay is a pre-transplant prognosis test for the risk of early acute rejection, while Tuteva™ is a post-transplant diagnostic focused upon acute cellular rejection. Both products aim to understand how a patient will and is responding to kidney transplantation.
Today, Verici confirmed that it has partnered with fourteen leading US, Australian and EU centres as part of the clinical validation study. Meanwhile, patient enrolment for both Clarava and Tuteva performance evaluation was completed ahead of schedule in August 2021.
It said this commitment shown is testament ‘to the recognition of the robust, scientific approach being taken by Verici to its data gathering and analysis, and to the shared desire to make much-needed and potentially very significant gains in transplant patient outcomes.’
Back in January 2021, Verici in-licensed further intellectual property (IP) relating to gene expression in a blood-based test to predict the risk of fibrosis or chronic kidney graft damage.
This is being commercialised under the name Protega and extends the Verici portfolio across the full transplant patient journey ‘by focusing on the prognosis of longer-term graft failure.’
Verici said Protega™ augments its portfolio ‘into an end-to-end transplant testing suite, from pre-transplant and short-term post-transplant to also include later-stage risk assessment.’
The Company said the current fourteen clinical trial sites participating in the validation study for Clarava™ and Tuteva™ will continue the enrolment of patients for an extended period for the validation study of Protega and is expected to reach target by the end of Q3 next year.
It said the potential to inform earlier clinical interventions with treatments such as anti-fibrotic therapies ahead of irreversible organ damage is another key step to improving outcomes.
Patti Connolly, Executive VP, Product Development of Verici Dx, said: “This is another step towards the commercialisation of our two lead products, and we look forward to the data analysis and reporting of key findings in 1Q22, with additional analyses to follow.”
We look forward to sharing the validation reporting in 2022 as we approach commercial readiness for our two lead products and continue with validation for our third product.”
In August 2021, Verici said patient enrollment for a multi-centre observational clinical validation study for its lead products had concluded ahead of management expectations.
Having completed its enrollment early, the developer of advanced clinical diagnostics for organ transplants said it remains on track to complete the validation study for these products by the end of 2021, in line with expectations set out in the Company’s admission document.
Verici Dx has partnered with eleven leading US and EU centres to date to run a global, non-randomised study for the clinical validation of its lead products, Clarava and Tuteva.
The Group’s Clarava™ assay is a pre-transplant prognosis test for the risk of early acute rejection, while Tuteva™ is a post-transplant diagnostic focused upon acute cellular rejection.
The study uses next generation sequencing in the Verici Dx lab to create transcriptomic profiles to validate performance characteristics of the lead Verici Dx signature tests.
Over the longer term, the study will also provide validation for a fibrosis test called Protega™. The patient enrolment process for Protega which is ongoing is expected to complete by 3Q22.
The end points of the study for this product are expected to be reached up to two years after the completion of enrolment, with data expected shortly thereafter around year-end 2024.
To date, enrolment is on target for completion of the Clarava and Tuteva validation study by the end of 2021 with data expected to be published ‘as soon as practicably possible’ in 2022.
Its efforts to accelerate the use of its organ transplant products comes at a time when the landscape of solid organ transplantation has dramatically changed as a result of COVID-19.
While developing, trialling and obtaining the necessary regulatory authorisations before launching medical technologies can be a lengthy process, Verici said it is focused on compressing these timescales with the aim to commercialise its kidney tests in 2022.
Follow News & Updates from Verici Dx: