SourceBio International: (SBI ) said it has secured a COVID-19 contract whereby the Group will provide testing to the England Rugby squad as part of the Six Nations tournament.
The Group, which provides integrated lab services and products internationally, said it has entered into an agreement with the Rugby Football Union (“RFU”) and Premiership Rugby Limited to provide its COVID-19 screening testing services for the England Rugby team.
Under the agreement, the Group will provide its COVID-19 testing to England Rugby Senior Men’s squad and support staff and to all 12 Premiership Rugby clubs players and staff.
The RFU detailed that it is also looking to roll-out this testing service to RFU Championship clubs who will be able to use this agreement to access the services provided by SourceBio.
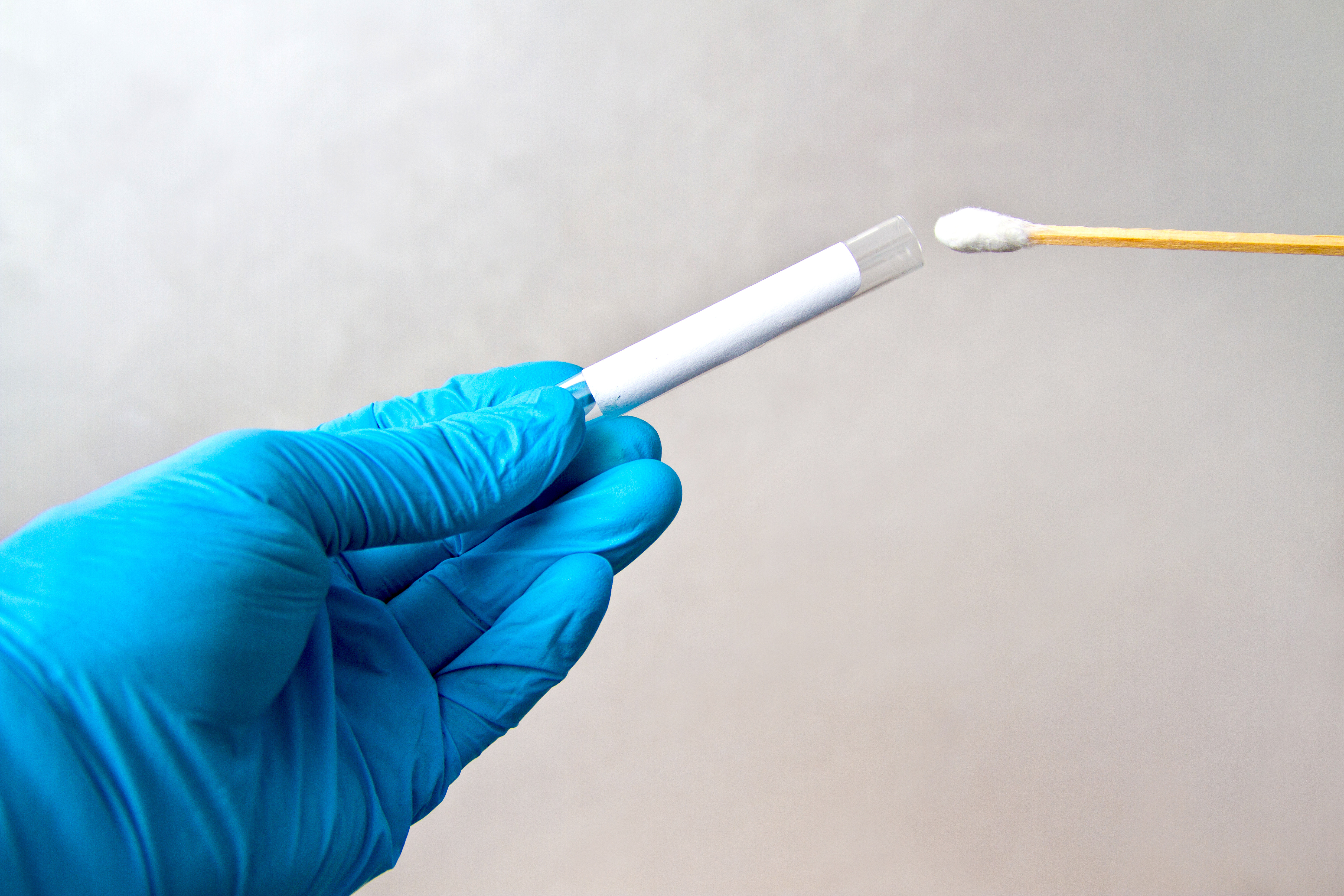
SourceBio said it will provide COVID-19 testing to both players and staff around training schedules and ahead of match days which will be turned around in less than 24 hours.
Third party healthcare professionals sourced by the Group will take swabs which will then be couriered to its ISO 15189 accredited laboratory facility in Nottingham for PCR Testing.
To date, SourceBio has processed more than 1 million COVID-19 antigen RT-PCR tests for the UK Government’s Department of Health and Social Care (DHSC), the NHS, and Private Hospital Groups and Commercial customers since first offering its services in May 2020.
"We are very pleased to support elite rugby return to play protocols using our state-of-the-art laboratories to allow elite rugby inEngland to continue to safely operate within current social distancing measures,” commented Jay LeCoque, Executive Chairman of SourceBio.
He added, “We have been at the forefront of COVID-19 testing programmes since the beginning of the pandemic, supporting the NHS and private healthcare groups, but also helping many of our commercial clients to maintain a safe working environment and helping businesses to get back on track.”
Today’s news is positive for both shareholders and rugby fans alike. Testing is essential to maintain the safety for players and rugby staff as demonstrated by the recent postponement of the France Vs Scotland 6 Nations clash due to the outbreak of COVID in the French camp.
Following a string of recent COVID-19 related agreements, signed since the start of 2021, the Group has already secured three testing clients this year resulting in the Board’s reiterating expectations for ‘significant earnings growth for 2021’.
This latest contract in the UK also follows news last week that it is to expand its stability storage and genomics operations in San Diego in California. This move will expand the health-grade capability to process 5,000 PCR tests/day and will enable additional opportunities for further growth. The extra capacity should be fully operational in Q2'21. Shares in SourceBio International have increased by over 10% since the start of the year.
Reasons to SBI
SourceBio International is an international provider of laboratory services to clients in the pharmaceutical industry, the NHS and to private healthcare providers. The Group is headquartered in Nottingham, with additional facilities in the UK, Ireland and the US.
The company saw a positive start on its first day of trading on AIM, adding around £8m to its initial £120m market capitalisation while the £35m raised at 162p is intended to be used by it to scale up COVID-19 testing capacity as well as paying off shareholder and bank loans.
“We are delighted by the strong support we’ve received from institutional investors. Our IPO on AIM allows us to significantly increase our COVID-19 testing capacity, accelerate earnings growth in our core business and execute on potential M&A opportunities,” said LeCoque.
He said at the time of the IPO: “It’s an exciting time for our business and we look forward to executing on our ambitious growth plans and delivering value to our shareholders.”
In November 2020, SourceBio unveiled that its funds from its IPO were enabling it to further scale its COVID-19 testing services to deliver against expected increases in future testing.
SourceBio previously forecasted total revenue of around £50m (FY19: £21.2m) and EBITDA of c.£14m (FY19: £3.0m) for the year ending 31 December 2020, with the vast majority of this increase in expected earnings driven by the contribution of COVID-19 testing revenues.
The group said in November 2020 that it had been accepted into the Increasing Capacity Framework Agreement for cancer testing services to NHS England, designed to reduce the significant backlog of elective surgeries impacting the NHS due to the pandemic which is expected to support the growth of the Healthcare Diagnostics business unit in 2021.
SourceBio entered into a strategic commercial partnership with Oxford Nanopore Technologies to offer a COVID-19 testing solution to corporate customers at scale via its own lab facilities. Last month, the group entered into a supply agreement to provide ‘state of the art’ products and lab services to an unnamed high street retail and pharmacy group.
Since December 2020, SourceBio has been supplying COVID-19 antigen RT-PCR testing services to the DHSC. It is currently awaiting a potential formal award under Public Health England's National Microbiology Framework in relation to further COVID-19 testing services.
For more news and updates on SourceBio International: